BIOS5004 Microbiology Assignment
Testing new antiviral candidates for SARS-CoV-2 Coronavirus
Scenario
You are employed by a pharmaceutical company, where all research in your Department has recently been redirected to explore new antiviral candidates for the inhibition of SARS-CoV-2coronavirus and hence the treatment of Covid-19 patients. Asone of the virologists, you have been searching for promising compounds.
BIOS5004 Microbiology Assignment

BIOS5004 Microbiology Assignment
Background
Several drug candidates have been explored over the past 2 years, for treatment of severe Covid-19 (hospitalized) cases, including two that have shown particular promise:
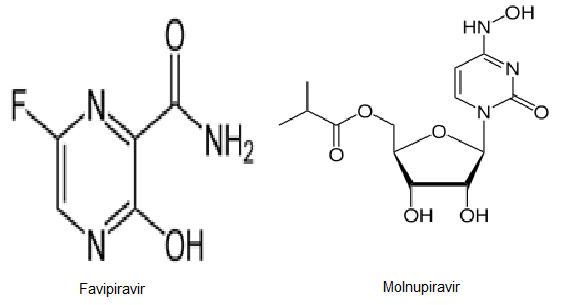
Favipiravirisa nucleoside analogue that can act as a mimic of both guanosine and adenosine. It has been documented as having inhibitory effects against a range of viruses that have an RNA genome. It is not currently licenced for use against Covid-19.
Molnupiravirisa synthetic nucleoside derivative that gained approval for medical use in the UK in November 2021 and in the US a month later. However, the approval is only for emergency use in certain populations, because the drug’s efficacy is limited and there are concerns that the mechanism of action of molnupiravir could inadvertently prolong the COVID-19 pandemic.
BIOS5004 Microbiology Assignment
Because of the limitations to the use of molnupiravir, your companyis keen to find alternatives that can be used safely and effectively in all cases of severe Covid-19 disease.
However, your company will only receive approval to pursue clinical trials with compounds that show statistically significant superior antiviral effects compared to molnupiravir.
Preliminary screening by your company, of over 10,000 compounds in their archives, has highlighted three drug compounds that have demonstrate dinhibition of SARS-COV-2 in basicin vitrotests.
You now intend to test these three compounds more fully, in a cell-based assay that will allow you to consider the level of virus inhibition in direct comparison with molnupiravir.
BIOS5004 Microbiology Assignment
Experimental work
You are using a cell line known as Vero/hSLAM(Ono et al., 2001[1]) which you have sourced from the ECACC General Cell Collection (part of Public Health England), and which is fully permissive for SARS-CoV-2 replication.
You routinely culture these cells as monolayers, which means that they adhere as a single layer of cells on the inside wall of a specialised tissue culture (plastic) flask.
The cells grow under a thin depth of nutrient liquid, known as a culture medium. Your use a medium calledEarle’s Minimum Essential Medium (EMEM) which must be supplemented withFetal Bovine Serum (FBS) to a final concentration of 7% FBS, to maintain healthy cells and to promote mitosis.
The cells grow best at 37°C and in an incubator with a 5% CO2 atmosphere to maintain the pH of the cells and medium as they metabolise nutrients and release by products.
Below is an outline of the experiment – read it carefully and then answer the questions at the end.
Submit your work as a single document, into the Turnitin link on the module Moodle page.
BIOS5004 Microbiology Assignment
Day 1 – routine cell culturing
You have been maintaining monolayer cultures of Vero/hSLAM cells in small plastic cell culture flasks. You begin your experiment by establishing enough of these flasks to have plenty of cells to work with. Everything that you do is performed in a sterile laminar flow hood, and you practice high-level aseptic technique.
- Taking cells from a flask that has been set up a few days ago, you replace the culture medium in the flask with 3 mL of phosphate-buffered saline (PBS).
- You scrape / dislodge the cells from the flask wall into this solution. PBS is an isotonic solution that preserves the cells for a short period of time.
- You count a sample of the cells (suspended in PBS) using a haemocytometer, to determine the cell density of this temporary cell suspension.
- Using this cell density, you calculate the volume of cell suspension that you would need to add to a new flask, to establish a starting culture containing 4 x 105 cells per flask.
- You add 20 mL of FBS-supplemented EMEM, mixing these components together before you add them.
- You set up 3 of these flasks and incubate the 3 new cultures at 37°C in an incubator with a 5% CO2 atmosphere, checking them daily for cell growth.
BIOS5004 Microbiology Assignment

BIOS5004 Microbiology Assignment
Day 4 – establishing the test cultures
The cells reach over 95% confluence by Day 4, meaning they are ready to use. You use one of the flasks to repeat the procedure above, in order to keep the cell linegoing (i.e.you set up 3 new flasks, just as you did on Day 1).
- Taking the other TWO flasks that you set up on Day 1, you dislodge the cells from each flask into 3 mL of PBSand combine them so that you have a single cell suspension of ~6 mL volume.
- You count a sample of the cells (suspended in PBS) using a haemocytometer, to determine the cell density of this temporary cell suspension.
You aim to set up a series of test culture plates that allow you to make small, replicate cultures in a series of wells.
o Each test culture plate has 24 wells for cell culture
o You want to set up 4 of these 24-well culture plates
o Every culture well needs to have 2 x 104 cells added
o Every culture well needs 1 mL volume in total.
You intend to make a single “master mix” cell suspension to do this, to promote accuracy, consistency and to minimise pipetting errors.
This master mix must contain the right number of cells to allow you to set up all your test cultures in one go and the right volumes of EMEM and FBS to allow you to set up all your test cultures in one go
In other words, you need to make just one single mixture of cells, EMEM and FBS, from which you simply pipette 1 mL into every well of the four 24-well plates
Once the test plates are set up, you incubate the test culture plates at 37°C and in an incubator with a 5% CO2 atmosphere, for 2hrto allow the cells to adhere to the bottom of the wells.

BIOS5004 Microbiology Assignment
Day 5 – Infecting cultures with virus and testing candidate antivirals
After you incubate the test cultures for 2hr, you infectevery well (i.e. every monolayer culture) with SARS-CoV-2 at an MOI of 2.0 pfu / cell. Once the cells are infected, you incubate them in the presence of different drugs.
- The right volume of virus, calculated to achieve the required MOI, is added to every well of all the cell culture test plates.
- The plates are incubated at 37°C for 2hr, to allow the virus to attach to and penetrate the cells.
- The inoculum (the medium containing the virus) is removed from every well and the monolayers are quickly washed with medium containing no virus.
- 1 mL of fresh FBS-supplemented EMEM media is added to every well, either molnupiravir or one the 3 candidate compounds you have found promising in earlier tests.
- Each compound is tested, across a 24-well plate, at 3 concentrations, meaning that each concentration is tested in 6 wells.
- Each plate contains a negative control, which comprises 6 wells incubated with FBS-supplemented EMEM containing no drug.
The layout for each test plate is as follows. Remember, every well contains a known number of cells and a known amount of virus. All that differs between the wells is the concentration of the drug. Each test plate (there are 4) is set up with one of the drugs.
BIOS5004 Microbiology Assignment
Day 8–harvesting virus and the plaque assay
The cells are incubated for 3 days at 37°C and in an incubator with a 5% CO2 atmosphere to maintain the pH of the cells and medium.
The liquid medium of each well is harvestedand stored at 4°C. Over the coming weeks, you will test every harvested sample,using a plaque assay, to determine the amount of virus that has been released from the infected cell cultures. You hope that one of your test drug candidates will have had a significant effect on the amount of virus released.
Remember:
- there were 4 drugs tested (molnupiravir and your 3 unknown drug candidates)
- each drug was tested at 3 different concentrations
- each concentration was tested using 6 replicate wells.
BIOS5004 Microbiology Assignment
That’s a lot of plaque assays! Each was done in the same way, as follows:
- A 24-well plate of test cultures was set up, using the same cell numbers as for Day 4.
- The harvested medium to be tested for virus (released from infected cultures) was diluted in 10-fold series up to 10-9. Dilutions were made in EMEM, just before use.
- Each virus dilution was tested in triplicate, by infecting 3 wells (3 cultures) with 0.1 mL of the diluted virus.
- After 2hr, this virus inoculum (0.1 mL) was removed and replaced with 1 mL of FBS-supplemented EMEM containing 1 % molten agarose, which was allowed to set.
- The culture plates were incubated for 3 days at 37°C and in an incubator with a 5% CO2 atmosphere.
At the end of this period, the cell monolayers were stained in order to highlight virus plaques, and these were counted manually and recorded.
For each plaque assay, the mean virus titre was determined (using the 3 replicates), and the results were recorded – you will find the virus titres on the following page.
BIOS5004 Microbiology Assignment
To be continue…..